Inhalation chamber for paediatric or adult mechanical ventilation circuit
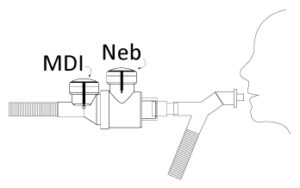
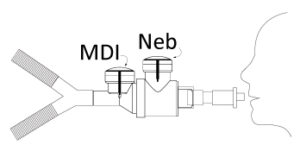
A low volume inhalation chamber developed to be used within both invasive and non-invasive mechanical ventilation circuits for pediatric care. MinimHal® inhalation chamber allows the use of pMDI and vibrating mesh nebulizer to be used simultaneously.

The MinimHal® inhalation chamber characteristic
Aimed to be used in a mechanical ventilation circuit, the MinimHal® inhalation chamber improves the drugs deposition into the lungs of patients under respiratory assistance. This device is a substitute to the T piece and allows the use of pMDI and vibrating mesh nebulizer simultaneously. Thanks to its convertor, MinimHal can be used on both paediatric and adult ventilation circuits.
Device description and intended use
MinimHal® is an inhalation chamber which can be plugged in different positions into mechanical ventilation circuits (invasive or non-invasive). The low volume of the MinimHal® allows it to be used in pediatrics. This new device improves lung deposition. It also helps the caregivers by reducing the number of ventilation circuits handlings while changing the type of drugs delivery (pMDI and nebulizer).
Whose is MinimHal® aimed for ?
The MinimHal® inhalation chamber has been designed for young and adult patients who need to be treated by inhaled therapy through a respiratory assistance. It is used to administrate inhaled bronchodilators, anti-inflammatory and antibiotics for ventilated patients in reanimation services, home care, intensive care or under respiratory assistance.
Operating principles of the MinimHal® inhalation chamber
MinimHal® is intended to be included in several locations in a mechanical ventilation circuit (invasive or non-invasive). MinimHal® can be used as well on paediatric as on adult circuits.
MinimHal® can be placed on inspiratory branch, before the Y piece.
MinimHal® can be placed between the Y piece and the patient interface.
When using MinimHal® after the Y-piece, make sure that the dead volume (plus the chamber volume – 60ml) is compatible with the patient’s tidal volume and, if necessary, remove MinimHal® from the circuit between treatment sessions.
Download the MinimHal® user manual
A possible use with the Respimat® inhaler
Respimat® (Boehringer Ingelheim) is an inhaler which is used for asthma treatment, obstructive chronicle Broncho pneumopathy and other respiratory infections.
The adapter can be provided with the inhalation chamber. It allows the use of Respimat® with MinimHal® on a mechanical ventilation circuit. It is designed to maintain the sealing on the circuit, the adapter allows a safe using.
Benefits of the use of MinimHal® inhalation chamber
MinimHal® allows to connect a vibrating mesh nebulizer and a pressurized metered dose inhaler with a single device in a mechanical ventilation circuit. MinimHal® provides significant benefits for ventilated patients and caregivers in critical care units.
1. MinimHal® can be used with both a pressurized metered dose inhaler (pMDI) and a vibrating mesh nebulizer. The assembly of the mechanical ventilation circuit is then simplified using one device instead of two.
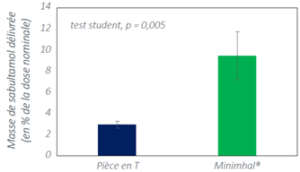
3. In vitro studies performed with the MinimHal® spacer showed a higher aerosol delivery with both the pMDI and the vibrating mesh nebulizer in comparison with T connectors, which are traditionally used. 4. Thanks to its low volume of 60 mL, MinimHal® can be used in both an adult and a pediatric mechanical ventilation circuit.
5. MinimHal®, when it is used with a vibrating mesh nebulizer or a pMDI, does not change the ventilators parameters.
6. Designed with a transparent material, MinimHal® make easier the monitoring of the mechanical ventilation circuit.
7. As it is made of a light material, MinimHal® does not weigh on the mechanical ventilation circuit. 8. Thanks to its specific adaptor, MinimHal® can be used with the soft mist inhaler (Respimat®).
Depostion of salbutamol in vitro with MinimHal® and an MDI adapter (Intersurgical) when using with a pressurised metered dose inhaler (ventoline®, GSK). The results refer to a test with the device placed on the inspiratory branch, in front of the T piece, with a paediatric mechanical ventilation model. The results are given as mean ± standard deviations (n=5).
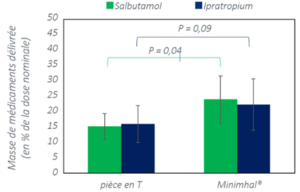
Used with a mesh nebulizer, MinimHal® increases pulmonary deposition of salbutamol.
Salbutamol and Ipratropium deposition in vitro with MinimHal® and the Aerogen Solo® T-piece used with the vibrating mesh nebulizer Aerogen Solo®. The result refer to as test with the device placed on the inspiratory branch, in front of the T piece, with a paediatric mechanical ventilation model. the result are given as mean ± standard deviation (n=5).
MinimHal® and COVID-19 : An appropriate spacer

If it not possible to switch from a pMDI treatment to a nebulized treatment, it is then recommended to use a vibrating mesh nebuliser. As its drug tank is isolated from the circuit, it enables to avoid the infection of the drug solution.
MinimHal® can be used with a pMDI as well as a vibrating mesh nebuliser. MinimHal® make it possible to go through a device to another, thus, avoiding to infect the patient environment.
Scientific publications
Aérosolthérapie chez les nouveau-nés : Comparaison des performances in vitro d’une chambre d’inhalation sans volume mort à un nébuliseur pneumatique.
CFA 2022 : M. Eckes, B. Hervieu, L. Fontaine, N. Duval, Q. Bequet, F. Beaubras, T. Porée
Effet du volume de la chambre d’inhalation sur l’administration du médicament en ventilation mécanique : étude in vitro.
RDD 2019 : M. Eckes, T. Porée
CIPP 2019 : M. Eckes, T. Porée
CPAP 2019 : M. Eckes, T. Porée
CIPP 2019: M.Eckes, T. Porée
RDD 2019 : M.Eckes, T. Porée
CPAP 2019 : M. Eckes, T. Porée
RFMV 2020 : M. Eckes, B. Demouveaux, B. Hervieu, A. Paris, A. Vanlaeys, T. Porée