
Spacer for mechanical ventilation circuit
The CombiHaler® is 100% designed and manufactured in France. CombiHaler® spacer for mechanical ventilation and critical care (ICU) enables the use of both a vibrating mesh nebuliser such as Aerogen®, and a pMDI at the same time.

Specifications of the spacer CombiHaler®
CombiHaler® is a device aimed to improve respiratory drug deposition for ventilated patients in critical care (ICU). It was designed to enable the use of a pMDI and an Aerogen® together.
Description of the device and its intended use
CombiHaler® is a spacer designed to be integrated on a breathing device circuit in invasive or non-invasive ventilation. It enables the connection of a pMDI and/or an Aerogen® Pro or an Aerogen® Solo without intervention on circuit.
CombiHaler® is intended to administer drugs by both nebulizer and pressurized metered dose inhaler (pMDI). It limits in particular the impaction deposit or the substance in the spacer.
The CombiHaler® spacer is single use only and is meant to be used in hospital, in intensive care services or for home care.
Who is it for ?
It is intended for patients suffering from Chronic Obstructive Pulmonary Disease (COPD), asthma or chronic obstructive respiratory insufficiency in pulmonology, critical care or operating suite.
How does the CombiHaler® spacer work ?
The spacer is meant to be crossed by gas flows in a circuit of a device for mechanical ventilation.
CombiHaler® has four openings leading to its inside volume. The CombiHaler® spacer has two openings meant to be connected to the respirator circuit: one openings is meant for the way in of the gas flow and the other one is meant for the way out of the gas flow. CombiHaler® also has an opening to plug a pressurised metered dose inhaler and an opening to plug a nebuliser Aerogen® Pro or Aerogen® Solo.
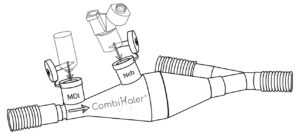
The openings to plug the pMDI and the nebuliser Aerogen® Pro or Aerogen® Solo are both formed in the upper part of the inhalation chamber. The positioning of the openings that are used to plug the pMDI and the nebulizer, not facing but one adjacent the other, make it possible to avoid the deposit related to the impaction of the particles, for example, produced by the Aerogen® Pro or Aerogen® Solo nebuliser on the inhaler or vice versa.
What are the benefits ?
Connect an Aerogen® on a ventilation circuit through a CombiHaler® provides significant benefits for ventilated patients and caregivers.
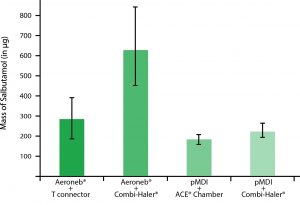
1. Save 50% of the nebulized drug: Used to be connected to an Aeroneb® Pro ou un Aerogen® Solo, CombiHaler® provides a pulmonary deposition of nebulized medication twice the traditional assembly with T piece (It is 40% with the Amikacine). Regarding the price of some drugs, it can represent a real benefit.
2. Using a CombiHaler® with an Aerogen® and a pMDI on a ventilation circuit simplifies it as it is possible to use only one device instead of two.
3. Using the CombiHaler® does not modify the respirator parameters
4. As it is transparent, CombiHaler® do not bother the monitoring of the ventilation circuit.
5. As a single patient device, CombiHaler® can be used during the lifespan of the ventilation system.

CombiHaler® and COVID-19 : an appropriate spacer

During the Covid-19 pandemic, it is recommended to use an inhaler. CombiHaler® enables and facilitates this use on ventilation circuits.
If it not possible to switch from a pMDI treatment to a nebulized treatment, it is then recommended to use a vibrating mesh nebuliser. As its drug tank is isolated from the circuit, it enables to avoid the infection of the drug solution.
CombiHaler® can be used with a pMDI as well as a vibrating mesh nebulizer. CombiHaler® make it possible to go through a device to another, thus, avoiding to infect the patient environment.
Scientifics publications
RFMV 2020 : M. Eckes, B. Demouveaux, B. Hervieu, A. Paris, A. Vanlaeys, T. Porée.
Effect of spacer volume on drug delivery using mechanical ventilation: An in vitro study.
RDD 2019 : M. Eckes, T. Porée.
ERS 2019 : M. Eckes, T. Porée.
In vitro evaluation of a new spacer with Respimat® in mechanical ventilation.
ISAM 2015 : N. Boukhettala , T. Porée , P. Diot , L.Vecellio.
In vitro evaluation of a spacer in a pediatric model of mechanical ventilation.
CIPP 2015 : N. Boukhettala, T. Porée.
In Vitro Performance of Spacers for Aerosol Delivery during Adult Mechanical Ventilation.
2014 [Epub ahead of print] : N. Boukhettala , T. Porée , P. Diot , L. Vecellio.
Study of the performances of a new spacer in mechanical ventilation.
ISAM 2013 : N. Boukhettala , T. Porée , P. Diot , L. Vecellio.
Study Of The Effectiveness Of A New Inhalation Chamber In Invasive Mechanical Ventilation.
ATS 2012 : N. Boukhettala, P. Diot, T. Porée, L. Vecellio.
A new Spacer for pMDI and nebulizers in mechanical ventilation.
ERS 2012 : N. Boukhettala, P. Diot, T. Porée , L. Vecellio.
Study Of The Effectiveness Of A New Inhalation Chamber In Invasive Mechanical Ventilation.
Abstract ATS 2012 : N. Boukhettala, P. Diot, L. Vecellio.
Study of the performances of a new spacer in mechanical ventilation.
N. Boukhettala , T. Porée , P. Diot, L. Vecellio.
Is Combihaler® usable for aerosol delivery in single limb non-invasive mechanical ventilation?
Hadeer S. Harb Bsc, Ahmed A. Elberry, Hoda Rabea, Maha Fathy, Mohamed E.A Abdelrahim.
The trademark Aerogen® belongs to The Aerogen Company Limited.
The trademark CombiHaler® belongs to ProtecSom-OptimHal SAS. Patents belong to Tour University, INSERM, and OptimHal-ProtecSom SAS together.




