CombiHaler® is an inhalation chamber designed and manufactured entirely in France for invasive and non-invasive mechanical ventilation circuits. It offers an effective and safe solution for improving medication administration via pressurized metered-dose inhaler and/or vibrating mesh nebulizer without disconnecting the circuit .


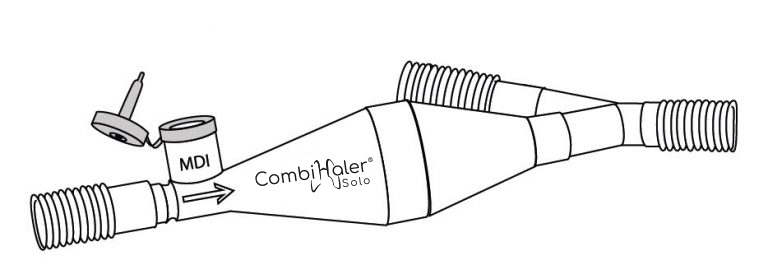
CombiHaler ® Solo has the same features as CombiHaler ® , except that it only allows the administration of medication by pressurized metered-dose aerosol.
CombiHaler® Solo and CombiHaler® were and operating room departments.
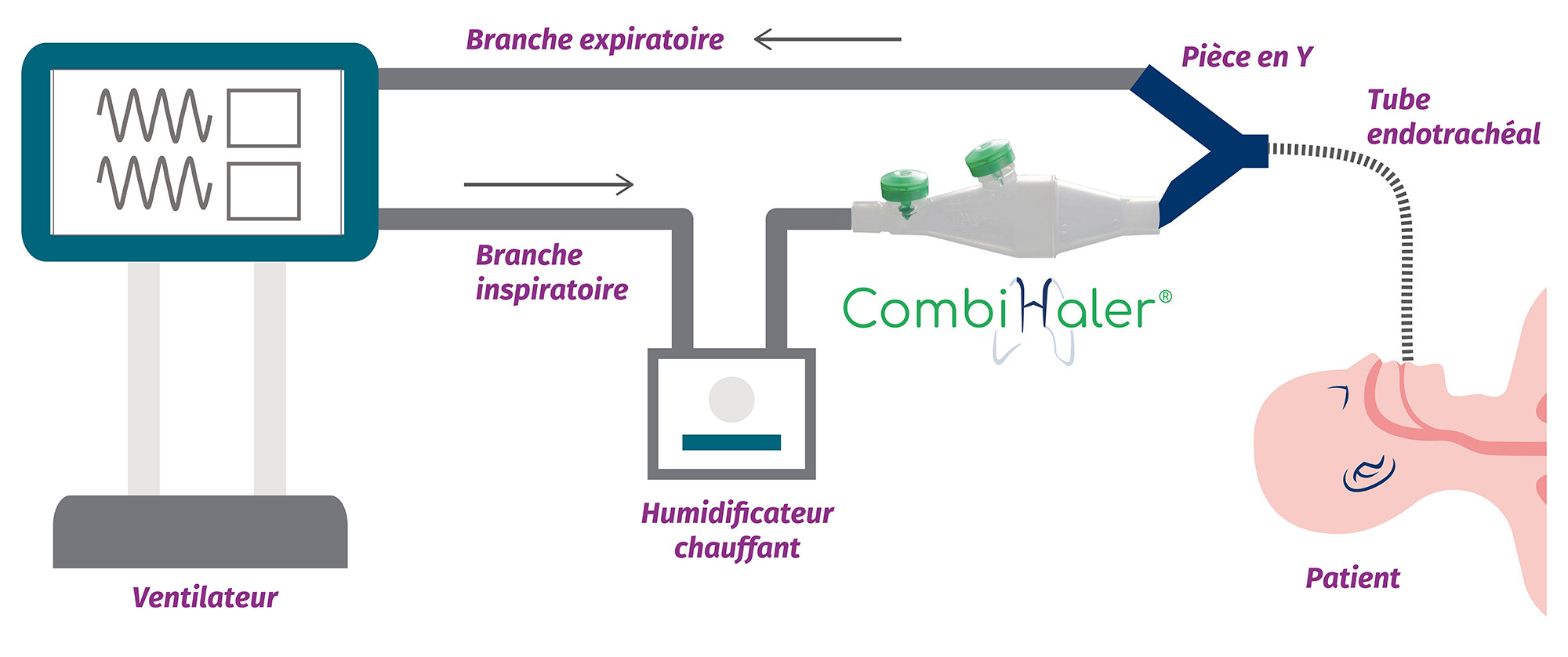
CombiHaler® Solo and CombiHaler® inhalation chambers have two openings designed to be connected to the ventilator circuit, one for the entry of a gas flow and the other for the exit of the gas flow.
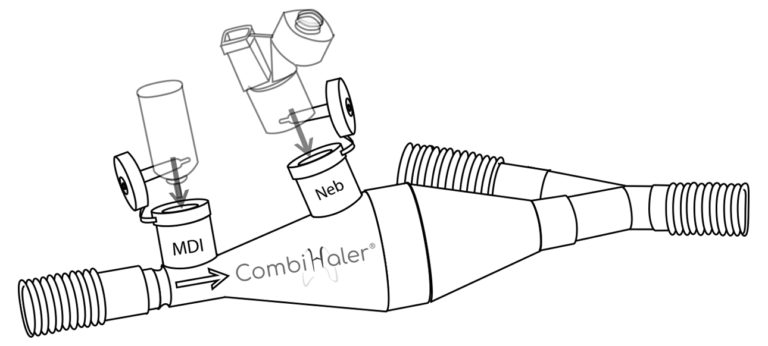
CombiHaler ® also includes an opening for receiving a pressurized metered-dose aerosol and an opening for receiving a sieve nebulizer.
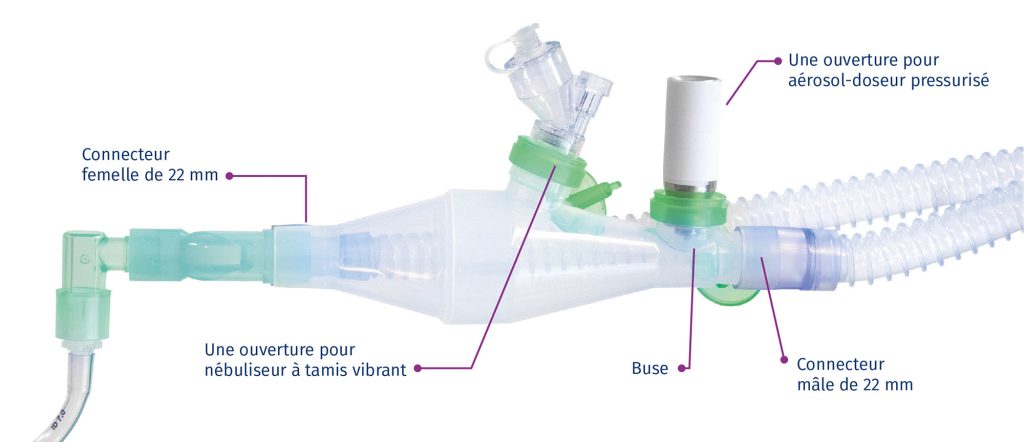
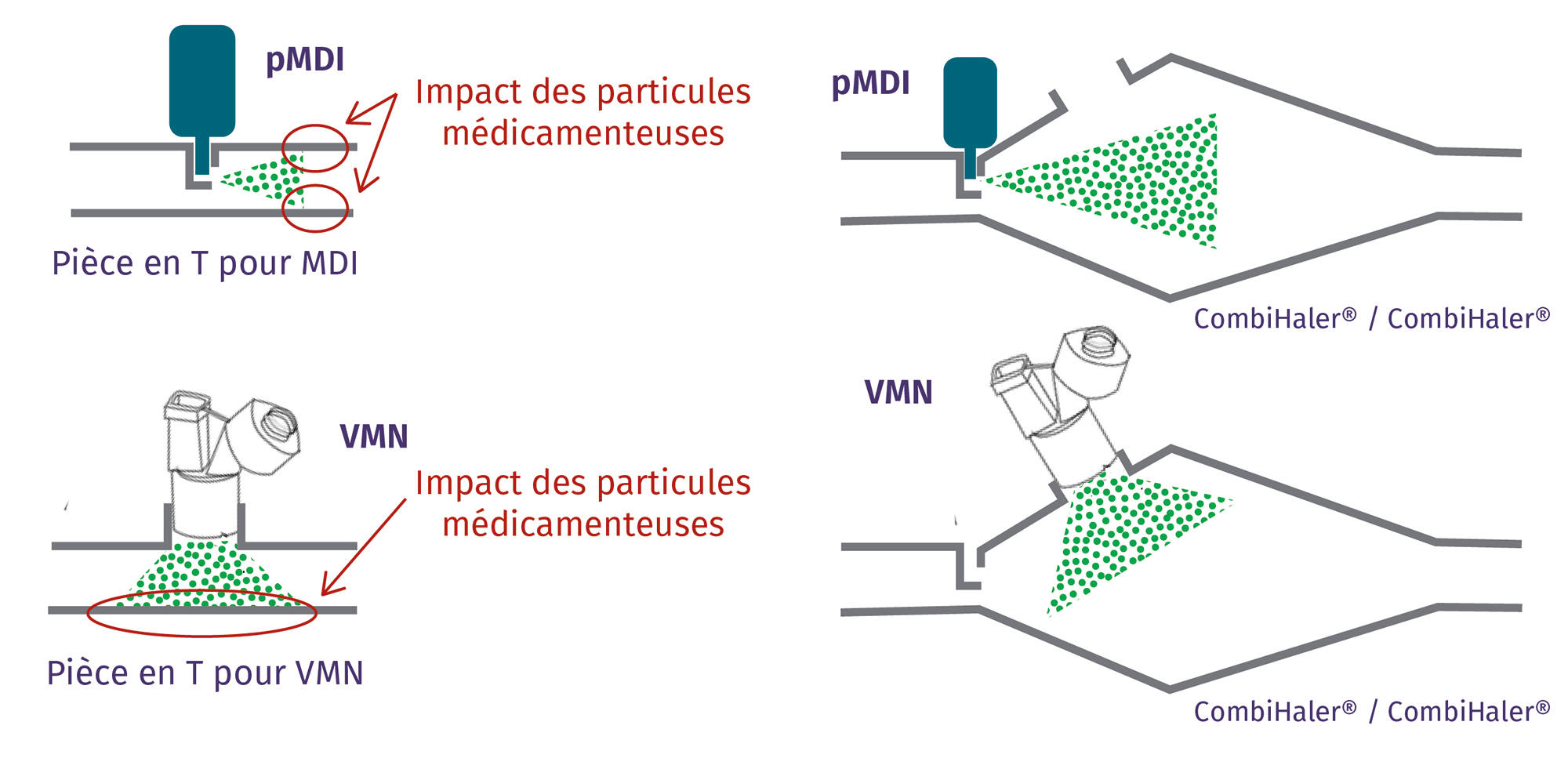
The shape of the CombiHaler® Solo and CombiHaler® reduces the impact of medication particles within the inhalation chamber, significantly reducing medication loss.
Respimat ® (Boehringer Ingelheim) is an inhaler used for the treatment of asthma, chronic obstructive pulmonary disease (COPD) and other respiratory conditions.
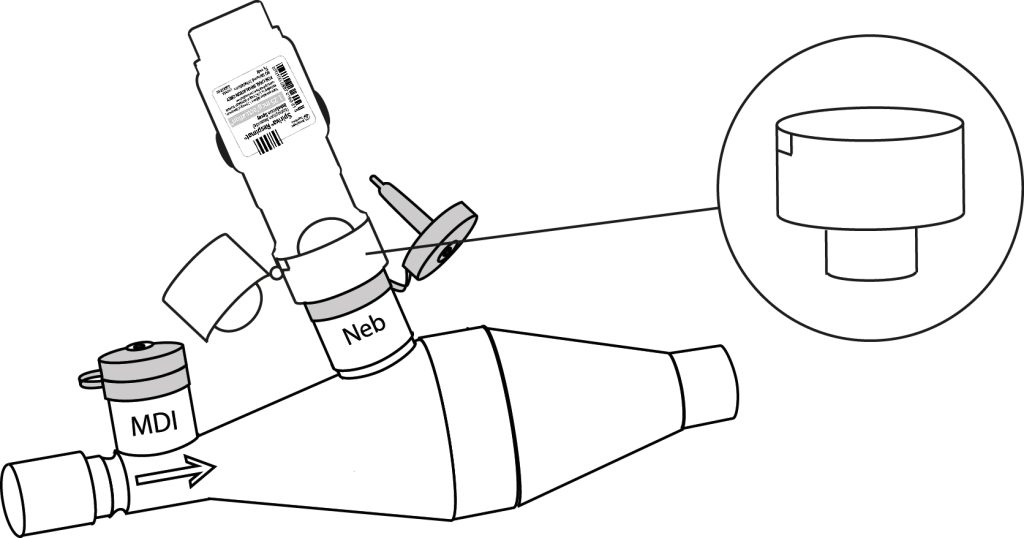
The adapter, which can be supplied with the spacer device, allows the use of a Respimat® inhaler with CombiHaler® on a mechanical ventilation circuit. Designed to maintain a tight seal on the circuit, the adapter ensures safe use.
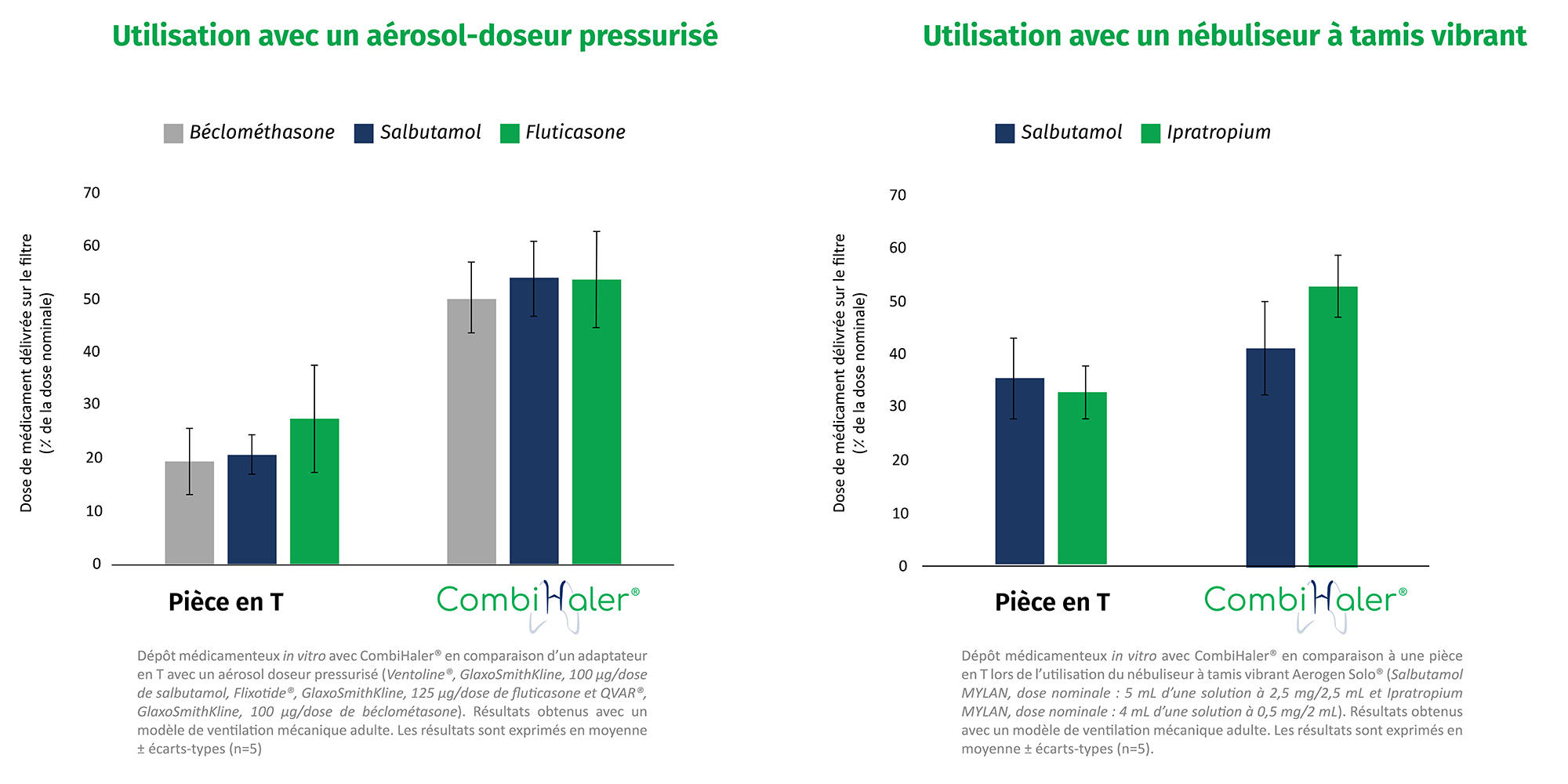
in vitro tests were conducted in our OptimHal laboratory. These tests proved that:
In vivo tests have proven that adding a preliminary dose of bronchodilator before nebulizing the drug significantly increases the effective pulmonary dose of the drug nebulized with CombiHaler®.¹
To learn more, access the CombiHaler Solo® and CombiHaler® .
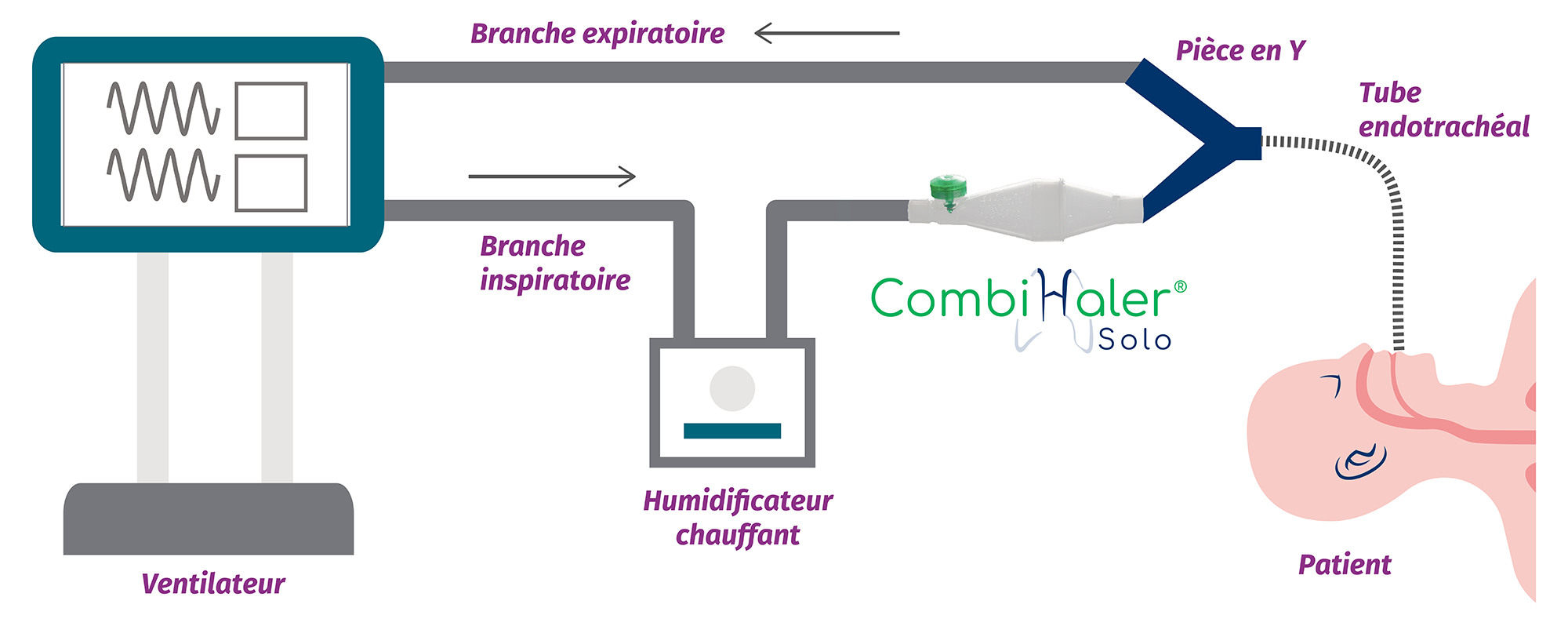
For installation in an adult mechanical ventilation circuit, CombiHaler ® Solo and CombiHaler ® connect to the inspiratory branch before the Y-piece.
CombiHaler® Solo and CombiHaler® are manufactured in France, under the ISO 13485:2016 standard, the international standard for medical devices. This certification demonstrates our commitment to adhering to extremely rigorous quality standards in the manufacturing of our devices. This means that our products are designed, manufactured, and tested with meticulous attention to detail to ensure the highest levels of safety and efficacy.

CombiHaler® Solo and CombiHaler® are inhalation chambers designed to serve the patient and facilitate the work of caregivers .
Discover in video how to connect our CombiHaler ® Solo and CombiHaler ® to an adult ventilation circuit before the Y room.